Tumor-on-chip technology validated to test the effect of a drug against glioblastoma
This technology makes it possible to evaluate the efficacy of drugs in biological models that represent at the microscale the conditions under which a tumor develops in the human body.
Research conducted in Lleida and Zaragoza has validated the use of cancer-on-a-chip technology to examine the effect of an anti-tumor drug (NNC-55-0396, a tetralol compound) against glioblastoma, the most frequent and aggressive brain cancer. Specifically, the Cell Signaling by Calcium research group of the Institute of Biomedical Research of Lleida (IRBLleida) and the University of Lleida (UdL), in collaboration with the Aragon Health Research Institute and the University of Zaragoza, has established the glioblastoma model on a chip. This research has been recently published in the journal Cell Death and Disease.
"This technology is interesting because it makes it possible to evaluate the efficacy of drugs in biological models that represent at the microscale the conditions under which a tumor develops in the human body," explained Judit Herreros, one of the heads of the Calcium Cell Signaling research group.
"Glioblastomes are very aggressive brain tumors, consisting of at least two different regions: a region where the tumor cells receive abundant nutrients and oxygen because they are well supplied by blood vessels, and at the same time a central area lacking vessels, where the cells have adapted to live in a hostile environment and lack of oxygen," added the researcher and professor at the UdL.
"The glioblastoma-on-chip is a chamber where glioblastoma cells are arranged reproducing these two regions. In this way, they are optimal models for studying the effect of chemotherapeutics, which reduces the need for preclinical studies in animals," defined the first authors of the article, Clara Bayona, researcher at the Aragon Institute for Health Research (IIS Aragón), and Lía Alza, researcher at IRBLleida and the University of Lleida, and Lía Alza, researcher at the University of Lleida and the University of Lleida.
"The results of this study demonstrate that the drug NNC-55-0396 has a predominant effect on the hypoxic region of the tumor, the cells in the center of the tumor. These results complement previous results of the group where the mechanism of action of the drug, which activates cellular stress pathways that lead to tumor cell death in culture, was described," said the other head of the Cell Signaling by Calcium group, Carles Cantí. "This current work brings us closer to what the drug could do in vivo," added the researcher and professor at the UdL.
The research has benefited from the experience of the Tissue Microenvironment Laboratory, one of the 15 groups of the Technologies and Innovation Applied to Health program of the IIS Aragón, the Center for Biomedical Research Network Bioengineering, Biomaterials and Nanomedicine (CIBER-BBN) and the University of Zaragoza. The group is led by Iñaki Ochoa and Sara Oliván and works on microfluidic technology (the technology involved in the movement of small quantities of liquids through very narrow channels) which has allowed the chips to be developed.
This research has been possible thanks to funding from the European Union's Horizon 2020 research and innovation program, a MINECO grant from the Ministry of Economy, Trade and Enterprise, the European Regional Development Fund and the Challenges program of the Ministry of Science, Innovation and Universities, the TV3 Marathon Foundation, a FINO-AGAUR grant and the Government of Aragon.
Article:
Bayona, C., Alza, L., Ranđelović, T. et al. Tetralol derivative NNC-55-0396 targets hypoxic cells in the glioblastoma microenvironment: an organ-on-chip approach. Cell Death Dis 15, 127 (2024). https://doi.org/10.1038/s41419-024-06492-1
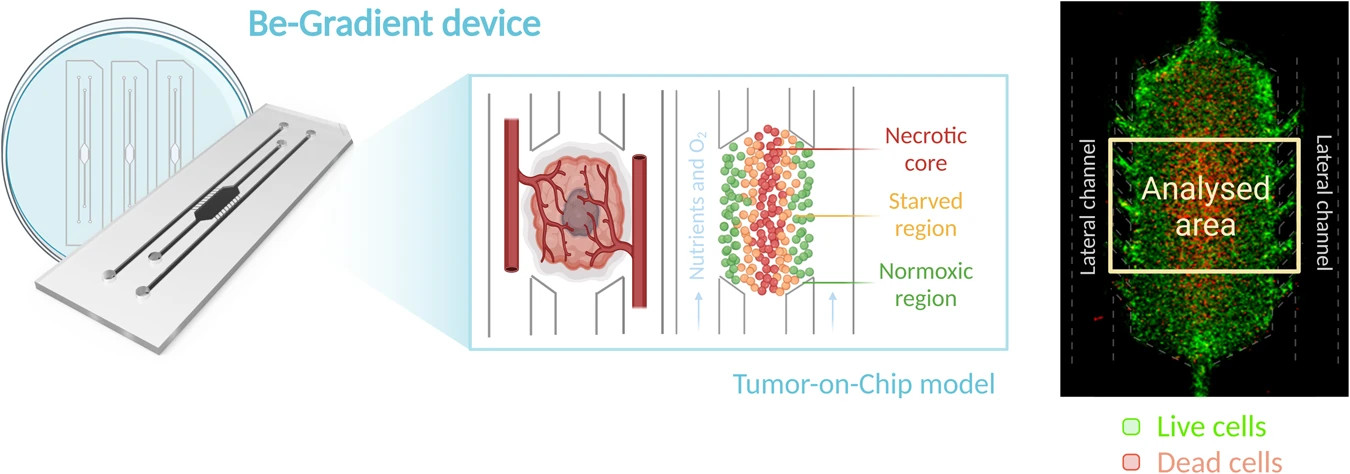
The image on the right shows a microdevice viewed through a fluorescence microscope [*2x]. Live cells are marked in green and dead cells in red. The dashed yellow square exemplifies the analysis area chosen for processing.

The research group Cell Signaling by Calcium






